Qsymia Engage: Home Delivery Pharmacy Program
I authorize Medvantx and its contractors and business partners (Medvantx) to use and disclose my personal information or the personal information of the individual under my care. I understand that such personal health information may include name, address, telephone number, email address, birthdate, information about health conditions, treatment and product information, treatment dates, eligible treatment type, medical history and general health, healthcare plan benefits and coverage, information about adherence to treatment, and other relevant personal and health information about me or about the individual under my care (“Personal Health Information”). I further understand that such information may be disclosed to VIVUS for the following purposes:
(1) to operate, administer, enroll me or the individual under my care in, and/or continue my participation or the participation of the individual under my care in the Qsymia Engage: Home Delivery Pharmacy Program and/or any other affiliated patient support services and activities related to my condition or treatment or the condition or treatment of the individual under my care(for example, adherence programs, and disease management support); (2) to provide informational and promotional materials relating to VIVUS products and services or me or to the individual under my care, and/or my condition or treatment or the condition or treatment of the individual under my care; and/or (3) to improve, develop, evaluate, and continue VIVUS products, services, materials, and programs related to my condition or treatment or the condition or treatment of the individual under my care.
In order for Medvantx and VIVUS to provide the above services and programs to me or the individual under my care, I understand they will need my Personal Health Information or the Personal Health Information of the individual under my care.
I understand that Medvantx may receive remuneration from VIVUS in exchange for disclosing the Personal Health Information described herein and/or for using the information to contact me with communications about VIVUS products that have been prescribed to me of the individual under my care and other patient support services.
Medvantx may release my Personal Health Information or the Personal Health Information of the individual under my care in whatever form and through whatever media, including the internet, as required by the purposes set forth.
I further understand that once Medvantx discloses my Personal Health Information or the Personal Health Information of the individual under my care to VIVUS, it may no longer be covered by federal privacy regulations, and, therefore, could be redisclosed. However, VIVUS agrees to protect my Personal Health Information or the Personal Health Information of the individual under my care by only using and disclosing it as stated in this Authorization or as otherwise allowed or required by law.
I understand that I may receive a copy of this authorization or revoke this authorization at any time by emailing [email protected]. However, if I revoke this authorization, I understand I or the individual under my care may no longer qualify for the Qsymia Engage: Home Delivery Pharmacy Program and/or the other services described above. I further understand that if Medvantx is disclosing my Personal Health Information or the Personal Health Information of the individual under my care to VIVUS, revocation of this authorization will only prevent further disclosure of my Personal Health Information or the Personal Health Information of the individual under my care to VIVUS by Medvantx after they receive notice of revocation and will not affect any uses already made in reliance on my authorization.
I understand that this authorization is voluntary and I may refuse to sign it. My refusal to sign will not affect my ability or the ability of the individual under my care to obtain treatment or payment for treatment. But, I or the individual under my care may be ineligible to qualify for the Qsymia Engage: Home Delivery Pharmacy Program and the other programs and services set forth above.
I understand that this authorization for Medvantx to disclose my Personal Health Information or the Personal Health Information of the individual under my care will expire three (3) years from the date of my signature below, unless I notify Medvantx to terminate it earlier, or unless another date is required by state or other applicable law(s).
Electronic Authorization:
I understand that by signing below and by checking the “I agree” box, I am consenting electronically and providing legal authorization for Medvantx, including any affiliates, subcontractors, and/or agents of Medvantx to use and share, my Personal Health Information or the Personal Health Information of the individual under my care for the purposes described within the above authorization. I am also indicating that I am at least 18 years old and either the patient or legal guardian of the patient by checking the “I agree” box.
A copy of this authorization will be emailed to you upon successfully completing registration. You can print a copy of the email for your records or download and print a copy of this authorization from your Qsymia Engage profile page.
Risk of Birth Defects with Qsymia®
(phentermine and topiramate extended-release capsules), CIV
Please read the following important safety information about the use of Qsymia in patients who can become pregnant.
You are considered a patient who can become pregnant if this applies to you:
Talk to your healthcare provider to help decide what birth control options are best for you.
Please see the chart below to review birth control options.
Your Birth Control Options
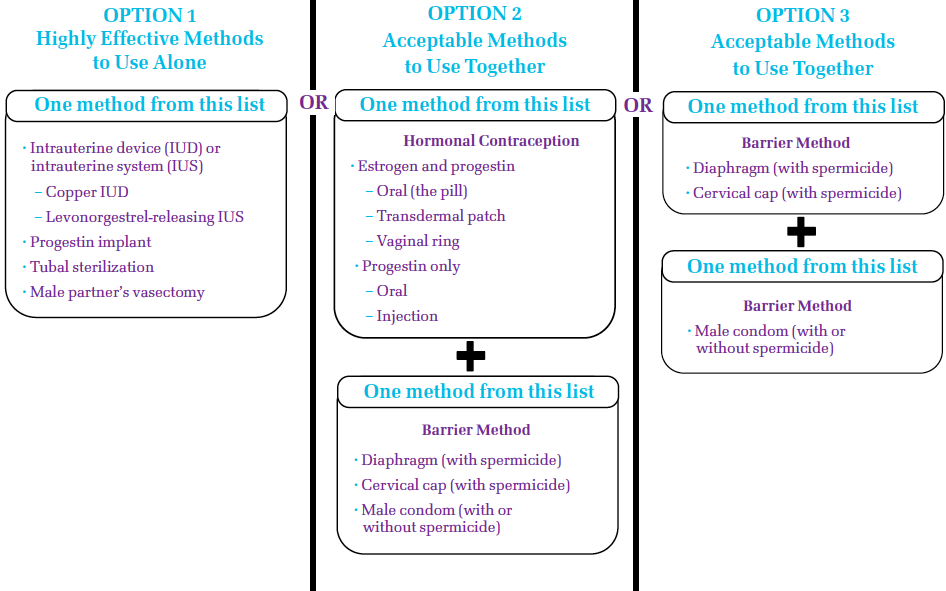
Keep in mind, even the most effective birth control methods can fail. But your chances of getting pregnant are lowest if the methods you choose are always used correctly and every time you have sex.
Please read the accompanying Qsymia® Medication Guide as it contains additional important safety information about your treatment. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about Qsymia, talk to your healthcare provider or pharmacist, contact VIVUS Medical Information at 1-888-998-4887, or visit the Web site www.QsymiaREMS.com.
![]()
©2024 VIVUS LLC. All rights reserved. 11/2024 RE-03-005-08
MEDICATION GUIDE
QSYMIA® (Kyoo sim ee’ uh)
(phentermine and topiramate extended-release
capsules), for oral use, CIV
What is the most important information I should know about QSYMIA?
QSYMIA can cause serious side effects, including:What is QSYMIA?
Who should not take QSYMIA? Do not take QSYMIA if you:
Before taking QSYMIA, tell your health care provider about all of your medical conditions, including if you:
Tell your health care provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. QSYMIA taken with other medicines may affect how each medicine works and may cause side effects.
Especially tell your health care provider if you take:
Ask your health care provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show your health care provider and pharmacist each time you get a new medicine. Do not start a new medicine without talking to your health care provider
How should I take QSYMIA?
If you take too much QSYMIA, call your health care provider or Poison Help line at 1-800-222-1222 or go to the nearest emergency room right away.
What should I avoid while taking Qsymia?
What are the possible side effects of QSYMIA?
QSYMIA can cause serious side effects, including:
Common side effects of QSYMIA in adults include:
Common side effects of QSYMIA in children 12 years and older include:
Tell your health care provider if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects of QSYMIA. For more information, ask your health care provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You can also report side effects to VIVUS at 1-888-998-4887.
How should I store QSYMIA?
Keep QSYMIA and all medicines out of the reach of children.
General Information about the safe and effective use of QSYMIA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use QSYMIA for a condition for which it was not prescribed. Do not give QSYMIA to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or health care provider for information about QSYMIA that is written for health professionals.
What are the ingredients in QSYMIA?
Active Ingredient: phentermine hydrochloride and topiramate extended-release.
Inactive Ingredients: FD&C Blue #1, FD&C Red #3, FD&C Yellow #5 and #6, ethylcellulose, gelatin, methylcellulose, microcrystalline cellulose, povidone, starch, sucrose, talc, titanium dioxide, and pharmaceutical black and white inks.
Copyright ©2024 VIVUS LLC. All rights reserved.
VIVUS LLC
900 E. Hamilton Avenue, Suite 550
Campbell, CA 95008 USA
US Patent Numbers: 7,056,890; 7,553,818; 7,659,256; 7,674,776; 8,580,298; 8,580,299; 8,895,057; 8,895,058; 9,011,905; and 9,011,906
Qsymia is a registered trademark of VIVUS LLC.
ME-03-001-11
This Medication Guide has been approved by the U.S. Food and Drug Administration
Revised: 11/2024